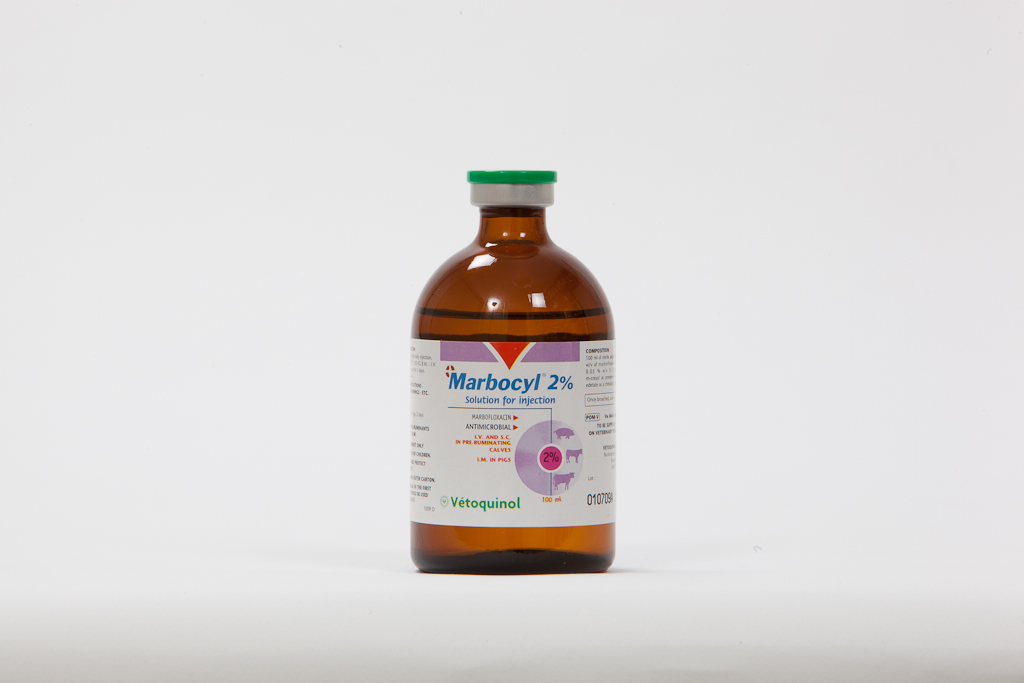
Marbocyl™ 2% Solution for Injection Species: Pigs, Cattle
Therapeutic indication: Pharmaceuticals: Antimicrobials: Injections
Active ingredient: Marbofloxacin
Product: Marbocyl™ 2% Solution for Injection
Product index: Marbocyl 2% Solution for Injection
Cattle – meat: 6 days
Pig – meat: 4 days
Presentation
Marbocyl 2% is presented as a sterile solution for injection.
Marbofloxacin 2.0% w/v
Disodium edetate 0.01% w/v
Thioglycerol 0.05% w/v
Metacresol 0.2% w/v
Uses
In pre-ruminant cattle up to 100kg bodyweight and pigs, Marbofloxacin 2 % is indicated in the treatment of respiratory infections caused by susceptible strains of organisms.
Dosage and administration
The recommended dosage is 2 mg/kg/day (1 ml/10 kg) in a single daily injection by subcutaneous or intravenous routes in cattle and by the intramuscular route in pigs.
Treatment duration is 3 – 5 days by the intravenous route in cattle,. 3 days by the subcutaneous route in cattle,. and 3 – 5 days by the intramuscular route in pigs.
The volume of injection should be limited to 10ml at each site of injection for pigs.
In order to reduce the risk of particulate contamination of the product, it is recommended that a draw-off needle be used to reduce the number of times the septum is punctured.
Contra-indications, warnings, etc
Special Precautions for use in Animals
Official and local antimicrobial policies should be taken into account when the product is used. Fluoroquinolones should be reserved for the treatment of clinical conditions which have responded poorly, or are expected to respond poorly, to other classes of antimicrobials. Wherever possible, fluoroquinolones should only be used based on susceptibility testing. Use of the product deviating from the instructions given in the SPC may increase the prevalence of bacteria resistant to the fluoroquinolones and may decrease the effectiveness of treatment with other quinolones due to the potential for cross resistance.
No severe side-effects are to be expected at doses up to 5 times the recommended dose in cattle and pigs. In particular no lesions of the articular joints are encountered.
Subcutaneous injection is well tolerated. Transitory inflammatory reactions are sometimes observed at the injection site, but without clinical impact.
Overdosage may cause acute signs in the form of neurological disorders which should be treated symptomatically.
Operator Warning
People with known hypersensitivity to fluoroquinolones should avoid using this product. Wash hands after use.
Withdrawal periods
Preruminating calves (up to 100kg bodyweight): 6 days
Pigs: 4 days
Pharmaceutical precautions
Following withdrawal of the first dose, use the product within 28 days. Discard unused material.
Do not store above 25°C. Protect from light.
Any unused veterinary medicinal product or waste materials derived from such veterinary medicinal products should be disposed of in accordance with local requirements.
Manure and slurry containing marbofloxacin should not be spread on the same area of land in successive years.
Packaging quantities
Packaged in amber type II glass vials of 10, 20, 50ml, 100 ml and 250ml.
The vials are closed with a chlorobutyl rubber stopper oversealed with aluminium caps.
Each vial is packaged in a cardboard box.
Not all pack sizes may be marketed.



Reviews
There are no reviews yet.